,由山东省医疗器械和药品包装检验研究院牵头修订的GB/T 14233.1-2022《医用输液、输血、注射器具检验方法 第1部分:化学分析方法》(以下简称“新版GB/T 14233. 1”)发布,并将于2023年11月1日正式实施。GB/T 14233.1是医用医输液、输血、注射器具检验方法的系列标准之一,主要规定了相关化学分析方法包含检验液的制备、常规溶出物试验方法以及材料中重金属元素分析方法、环氧乙烷残留量测试方法等。
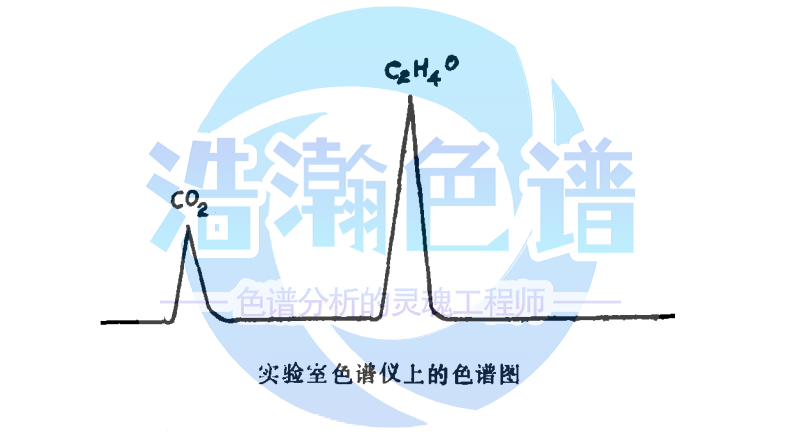
采用气相色谱法检测环氧乙烷残留量,制备供试液时有两种基本的样品浸提方法:模拟使用浸提法和极限浸提法。
环氧乙烷残留量用相对含量表示,计算公式为:CEO=5c/m,两个试验组检测结果均合格或不合格,以两次结果平均值作为最终检测结果。若两个试验组检测结果为一个合格,另一个不合格,需重新进行检测。
CEO—样品中环氧乙烷残留量,μg/g;
5—样品溶液体积,mL;
c—标准曲线上找出的供试液相对的浓度,μg/mL;
m—称样质量,g。
名称:毛细管柱
固定相:6%氰丙基苯基94%二甲基聚硅氧烷
规格:30m×0.25mm×1.4μm
型号:HH-624
应用:GB/T 14233.1-2022医用输液、输血、注射器具检验方法 第1部分:化学分析方法
GBT14233.1-2008医用输液、输血、注射器具检验方法第1部分:化学分析方法
GBT 14233.1-1998 医用输液、输血、注射器具检验方法 第1部分:化学分析方法
The revised GB/T 14233.1-2022 "Inspection Methods for Medical Infusion, Transfusion, and Injection Equipment Part 1: Chemical Analysis Methods" (hereinafter referred to as the "new version GB/T 14233 1) Released and will be officially implemented on November 1, 2023. GB/T 14233.1 is one of the series of standards for the inspection methods of medical infusion, blood transfusion, and injection equipment. It mainly specifies the relevant chemical analysis methods, including the preparation of test solutions, conventional dissolution test methods, analysis methods for heavy metal elements in materials, and testing methods for residual ethylene oxide.
Gas chromatography is used to detect the residual amount of ethylene oxide. There are two basic sample extraction methods for preparing the test solution: simulated extraction method and extreme extraction method.
The residual amount of ethylene oxide is expressed as relative content, and the calculation formula is: CEO=5c/m. The test results of both test groups are qualified or unqualified, and the average of the two results is taken as the final test result. If one of the two experimental groups passes the test and the other fails, retesting is required.
CEO - residual amount of ethylene oxide in the sample, μ g/g;
5- Sample solution volume, mL;
C - The relative concentration of the test solution found on the standard curve, in μ g/mL;
M - sample weight, g.
Name: Capillary Column
Fixed phase: 6% cyanopropyl phenyl 94% dimethyl polysiloxane
Specification: 30m × 0.25mm × 1.4 μ m
Model: HH-624
Application: GB/T 14233.1-2022 Inspection Methods for Medical Infusion, Transfusion, and Injection Equipment Part 1: Chemical Analysis Methods
GBT14233.1-2008 Inspection Methods for Medical Infusion, Transfusion, and Injection Equipment Part 1: Chemical Analysis Methods
GBT 14233.1-1998 Inspection Methods for Medical Infusion, Transfusion, and Injection Equipment Part 1: Chemical Analysis Methods
滕州市浩瀚色谱仪器技术服务有限公司
电话:0632-5667636
传真:0632-5667636
手机:15562228838,13963221227
地址:山东滕州市平行路(商务部和技术部)
邮箱:wangxiaoying9@126.com
QQ:1404939462
联系人:王经理
网址:www.haohansepu.com