药品中的残留溶剂系指在原料药物或辅料的生产中,以及在制制备过程中使用的,但在工艺过程中未能完全除去的有机溶剂)根据有机溶剂的毒性,国际上已统一将残留溶剂分为四类:第一类溶剂是应该避免使用的溶剂,毒性较大,一般为致癌物或危害环境的物质,共5种;第二类溶剂是应限制使用的溶剂,具有可逆毒性,对动物有非基因毒性致癌性,或不可逆的神经或致畸等毒性,共27种;第三类溶剂是毒性低,对人体危害较小的溶剂,共27种;第四类溶剂目前尚未有足够毒理学资料证明对人体有害,共10种。除另有规定外,第一类、第二类、第三类溶剂的残留限度应符合《中国药典》(2020年版)的规定;对其他溶剂(包括第四类)应根据生产工艺的特点,制定相应的限度,使其符合产品规范、《药品生产质量管理规范》(GMP)或其他基本的质量要求。
系统适用性试验
(1)用待测物的色谱峰计算,填充柱法的理论板数应大于1000;毛细管色谱柱的理论板数应大于5000。
(2)色谱图中,待测物色谱峰与其相邻的色谱峰的分离度应大于1.5。
(3)以内标法测定时,对照品溶液连续进样5次,所得待测物与内标物峰面积之比的相对标准偏差(RSD)应不大于5%;若以外标法测定,所得待测物峰面积的相对标准偏差(RSD)应不大于10%。
名称:毛细管柱
固定相:专用
规格:30m*0.53mm
型号:HH-TBS-30
应用:药典0861 残留溶剂测定法,理论塔板数
适用于:安捷伦,岛津,赛默飞,瓦里安,布鲁克,PE
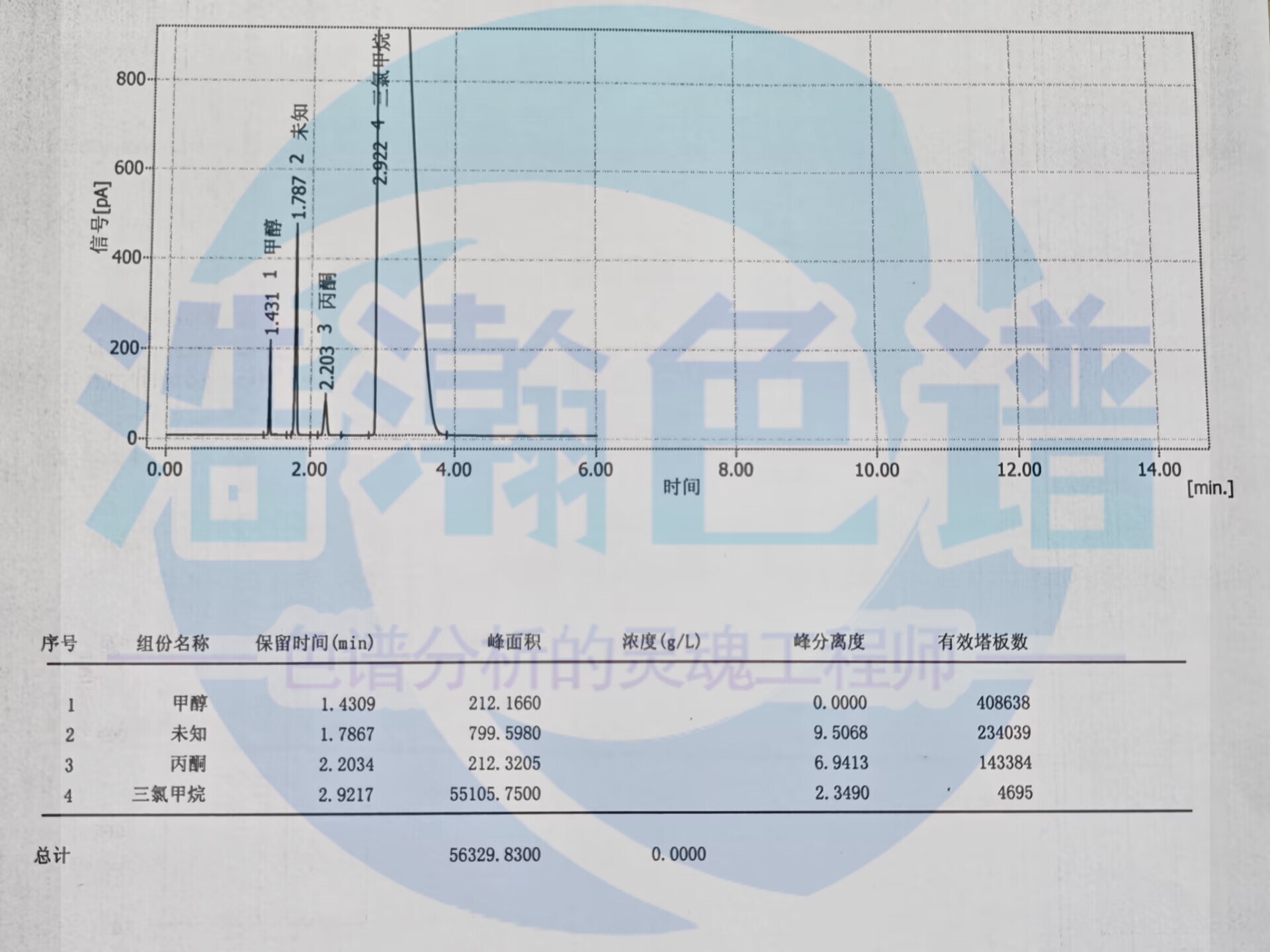
浩瀚色谱(山东)应用技术开发有限公司研制的《药典0861甲醇测定毛细管色谱柱理论塔板数》,柱效高,分离好,甲醇的理论塔板数可达408638,
丙酮的理论塔板数可达143384,满足药典溶剂残留;;塔板数的要求。

Residual solvents in drugs refer to organic solvents used in the production of raw materials or excipients, as well as in the preparation process, but not completely removed during the manufacturing process. According to the toxicity of organic solvents, residual solvents have been internationally classified into four categories: the first category of solvents should be avoided, which are highly toxic and generally carcinogenic or environmentally harmful substances, totaling five types; The second type of solvent is a solvent that should be restricted from use, with reversible toxicity, non genotoxic carcinogenicity to animals, or irreversible neurotoxicity or teratogenicity, totaling 27 types; The third type of solvent is low toxicity and less harmful to human health, with a total of 27 types; There is currently insufficient toxicological data to prove that the fourth type of solvent is harmful to human health, with a total of 10 types. Unless otherwise specified, the residual limits of Class I, II, and III solvents shall comply with the provisions of the Chinese Pharmacopoeia (2020 edition); For other solvents (including Class IV), corresponding limits should be established based on the characteristics of the production process to ensure compliance with product specifications, Good Manufacturing Practice (GMP), or other basic quality requirements.
System Applicability Test
(1) The theoretical number of plates for packed column method should be greater than 1000, calculated based on the chromatographic peak of the analyte; The theoretical number of plates for capillary chromatography columns should be greater than 5000.
(2) In the chromatogram, the separation degree between the chromatographic peak of the analyte and its adjacent peak should be greater than 1.5.
(3) When using the internal standard method for determination, the relative standard deviation (RSD) of the peak area ratio between the analyte and the internal standard obtained by continuously injecting the reference solution 5 times should not exceed 5%; If measured by external standard method, the relative standard deviation (RSD) of the peak area of the analyte obtained should not exceed 10%.
Name: Capillary Column
Fixed phase: Dedicated
Specification: 30m * 0.53mm
model: HH-TBS-30
Application: Pharmacopoeia 0861 Residual Solvent Determination Method, Theoretical Plate Count
Applicable to: Agilent, Shimadzu, Thermo Fisher, Varian, Brooke, PE
The "Pharmacopoeia 0861 Methanol Determination Capillary Chromatography Column Theoretical Plate Number" developed by Haohan Chromatography (Shandong) Application Technology Development Co., Ltd. has high column efficiency and good separation. The theoretical plate number of methanol can reach 408638,
The theoretical number of trays for acetone can reach 143384, which meets the requirements for residual solvents in pharmacopoeia;; Requirements for the number of tower plates.
滕州市浩瀚色谱仪器技术服务有限公司
电话:0632-5667636
传真:0632-5667636
手机:15562228838,13963221227
地址:山东滕州市平行路(商务部和技术部)
邮箱:wangxiaoying9@126.com
QQ:1404939462
联系人:王经理
网址:www.haohansepu.com